About Clinical Trials
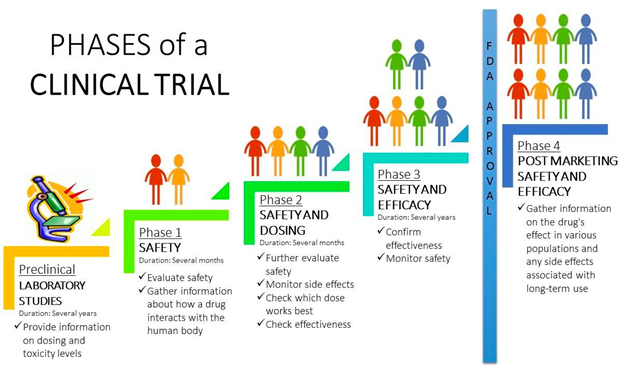
Clinical trials are a type of research study that helps find new ways to prevent or treat disease by evaluating their long-term safety and effectiveness in diverse populations. Clinical trials are typically conducted in 4 phases to provide answers to different questions about the safety and effectiveness of a drug or treatment being researched.
Phase I
Phase I trials answer the question, “is the treatment safe?”. If a trial is a phase I study, that means it’s the first time the treatment is being tested in a small group and helps to identify the most tolerable dose of the medication.
Phase II
Phase II trials answer the question, “does the treatment work?”. This phase of a trial further evaluates the safety and side effects of the treatment in a larger group of people and continues to research the most tolerable dose of the medication.
Phase III
Phase III trials answer the question, “is the treatment better than what’s currently available?”. This phase monitors the safety and side effects in a large group of people and looks at the effectiveness of the treatment compared to the standard treatment for the disease or a placebo (a placebo is identical to the study drug but contains no active ingredients).
Phase IV
Phase IV trials answer the question, “what more can researchers learn from this new treatment?”. This phase monitors the safety of the treatment over time and may involve up to thousands of participants in order to continue learning about how effective the treatment is over an extended period of time.
Clinical trials follow strict ethical principles, legal codes, and international legislation to ensure patient safety. Prior to joining a trial, every participant must review a document called an Informed Consent form with the research site to ensure the purpose of the study, the expectations of participation, and any possible side effects are understood. Research sites and study teams closely monitor each participant to ensure their safety and maintain open and honest communication throughout the trial. A participant may withdraw from the study at any time, for any reason.
All research sites follow procedures and safety measures provided to them in the study protocol. Study protocols are reviewed for patient safety and scientific content by an independent committee called an Institutional Review Board (IRB). Protocols are strictly adhered to by study sites and provide clear guidance on whether it is safe for a participant to continue in a trial or if they should be discontinued from the trial for their own safety. While a participant maintains the right to withdraw from a trial at any time without reason, discontinuing from a trial is ultimately the decision of the research site and is typically done when it is no longer safe for the participant to continue in the trial.
Clinical Trial Journey